What Animals Reproduce By Having Their Babies Grow Out Of Their Sides

The asexual, all-female whiptail species Aspidoscelis neomexicanus (center), which reproduces via parthenogenesis, is shown flanked by two sexual species having males, A. inornatus (left) and A. tigris (correct), which hybridized naturally to form A. neomexicanus.
Parthenogenesis (;[1] [two] from the Greek παρθένος , parthénos , 'virgin' + γένεσις , génesis , 'creation'[three]) is a natural form of asexual reproduction in which growth and evolution of embryos occur without fertilization by sperm. In animals, parthenogenesis ways development of an embryo from an unfertilized egg cell. In plants parthenogenesis is a component process of apomixis.
Parthenogenesis occurs naturally in some plants, some invertebrate fauna species (including nematodes, some tardigrades, water fleas, some scorpions, aphids, some mites, some bees, some Phasmatodea and parasitic wasps) and a few vertebrates (such equally some fish,[4] amphibians, reptiles[five] [6] and very rarely birds[seven] [viii]). This blazon of reproduction has been induced artificially in a few species including fish, amphibians, and mice.[ix] [10]
Normal egg cells class in the process of meiosis and are haploid, with one-half as many chromosomes as their female parent's body cells. Haploid individuals, however, are usually non-feasible, and parthenogenetic offspring usually have the diploid chromosome number. Depending on the mechanism involved in restoring the diploid number of chromosomes, parthenogenetic offspring may have anywhere between all and half of the female parent's alleles. The offspring having all of the mother's genetic material are called full clones and those having only half are called half clones. Full clones are usually formed without meiosis. If meiosis occurs, the offspring volition get simply a fraction of the mother's alleles since crossing over of Deoxyribonucleic acid takes place during meiosis, creating variation.
Parthenogenetic offspring in species that use either the XY or the X0 sexual activity-determination system take 2 X chromosomes and are female. In species that use the ZW sexual practice-determination system, they have either two Z chromosomes (male) or ii W chromosomes (mostly non-viable but rarely a female), or they could take ane Z and one Due west chromosome (female).
Parthenogenesis does non apply to isogamous or monogamous species.[11]
Life history types [edit]

A babe Komodo dragon, Varanus komodoensis, produced through parthenogenesis. Komodo dragons are an instance of a species which tin produce offspring both through sexual reproduction and parthenogenesis.
Some species reproduce exclusively by parthenogenesis (such as the bdelloid rotifers), while others tin switch betwixt sexual reproduction and parthenogenesis. This is chosen facultative parthenogenesis (other terms are cyclical parthenogenesis, heterogamy[12] [13] or heterogony[14] [15]). The switch between sexuality and parthenogenesis in such species may exist triggered by the season (aphid, some gall wasps), or by a lack of males or by conditions that favour rapid population growth (rotifers and cladocerans like Daphnia). In these species asexual reproduction occurs either in summertime (aphids) or as long as conditions are favourable. This is because in asexual reproduction a successful genotype tin can spread quickly without being modified past sex activity or wasting resources on male offspring who won't give nascency. In times of stress, offspring produced by sexual reproduction may exist fitter as they take new, perhaps beneficial gene combinations. In add-on, sexual reproduction provides the benefit of meiotic recombination between not-sister chromosomes, a process associated with repair of Dna double-strand breaks and other DNA damages that may be induced by stressful weather condition.[16]
Many taxa with heterogony take inside them species that have lost the sexual phase and are now completely asexual. Many other cases of obligate parthenogenesis (or gynogenesis) are found among polyploids and hybrids where the chromosomes cannot pair for meiosis.
The production of female offspring by parthenogenesis is referred to equally thelytoky (e.thou., aphids) while the production of males past parthenogenesis is referred to as arrhenotoky (east.yard., bees). When unfertilized eggs develop into both males and females, the phenomenon is chosen deuterotoky.[17]
Types and mechanisms [edit]
Parthenogenesis can occur without meiosis through mitotic oogenesis. This is called apomictic parthenogenesis. Mature egg cells are produced by mitotic divisions, and these cells directly develop into embryos. In flowering plants, cells of the gametophyte can undergo this procedure. The offspring produced by apomictic parthenogenesis are full clones of their mother. Examples include aphids.
Parthenogenesis involving meiosis is more complicated. In some cases, the offspring are haploid (e.thousand., male ants). In other cases, collectively called automictic parthenogenesis, the ploidy is restored to diploidy past various means. This is because haploid individuals are not viable in well-nigh species. In automictic parthenogenesis, the offspring differ from one some other and from their female parent. They are chosen half clones of their mother.
Automictic [edit]

Automixis [18] is a term that covers several reproductive mechanisms, some of which are parthenogenetic.[19]
Diploidy might be restored by the doubling of the chromosomes without cell sectionalization before meiosis begins or afterwards meiosis is completed. This is referred to as an endomitotic wheel. This may also happen by the fusion of the offset two blastomeres. Other species restore their ploidy past the fusion of the meiotic products. The chromosomes may non separate at ane of the two anaphases (chosen restitutional meiosis) or the nuclei produced may fuse or one of the polar bodies may fuse with the egg cell at some stage during its maturation.
Some authors consider all forms of automixis sexual as they involve recombination. Many others classify the endomitotic variants as asexual and consider the resulting embryos parthenogenetic. Amongst these authors, the threshold for classifying automixis as a sexual procedure depends on when the products of anaphase I or of anaphase II are joined. The benchmark for "sexuality" varies from all cases of restitutional meiosis,[twenty] to those where the nuclei fuse or to but those where gametes are mature at the fourth dimension of fusion.[xix] Those cases of automixis that are classified as sexual reproduction are compared to self-fertilization in their mechanism and consequences.
The genetic limerick of the offspring depends on what blazon of apomixis takes place. When endomitosis occurs earlier meiosis[21] [22] or when central fusion occurs (restitutional meiosis of anaphase I or the fusion of its products), the offspring get all[21] [23] to more than than half of the mother's genetic material and heterozygosity is mostly preserved[24] (if the mother has two alleles for a locus, it is likely that the offspring volition go both). This is because in anaphase I the homologous chromosomes are separated. Heterozygosity is not completely preserved when crossing over occurs in primal fusion.[25] In the case of pre-meiotic doubling, recombination, if information technology happens, occurs betwixt identical sister chromatids.[21]
If terminal fusion (restitutional meiosis of anaphase II or the fusion of its products) occurs, a little over one-half the mother's genetic material is present in the offspring and the offspring are mostly homozygous.[26] This is considering at anaphase II the sister chromatids are separated and whatever heterozygosity is present is due to crossing over. In the case of endomitosis later on meiosis, the offspring is completely homozygous and has simply half the female parent's genetic fabric.
This can result in parthenogenetic offspring existence unique from each other and from their mother.
Sex of the offspring [edit]
In apomictic parthenogenesis, the offspring are clones of the mother and hence (except for aphids) are ordinarily female. In the case of aphids, parthenogenetically produced males and females are clones of their mother except that the males lack 1 of the X chromosomes (XO).[27]
When meiosis is involved, the sexual practice of the offspring will depend on the type of sex decision organisation and the type of apomixis. In species that use the XY sex-determination system, parthenogenetic offspring volition take 2 X chromosomes and are female. In species that use the ZW sex-determination system the offspring genotype may be i of ZW (female),[23] [24] ZZ (male), or WW (non-viable in nearly species[26] just a fertile,[ dubious ] feasible female in a few (due east.g., boas)).[26] ZW offspring are produced by endoreplication before meiosis or by central fusion.[23] [24] ZZ and WW offspring occur either past terminal fusion[26] or by endomitosis in the egg prison cell.
In polyploid obligate parthenogens similar the whiptail cadger, all the offspring are female person.[22]
In many hymenopteran insects such as honeybees, female eggs are produced sexually, using sperm from a drone father, while the production of further drones (males) depends on the queen (and occasionally workers) producing unfertilized eggs. This ways that females (workers and queens) are always diploid, while males (drones) are always haploid, and produced parthenogenetically.
Facultative [edit]
Facultative parthenogenesis is the term for when a female tin produce offspring either sexually or via asexual reproduction.[28] Facultative parthenogenesis is extremely rare in nature, with just a few examples of animal taxa capable of facultative parthenogenesis.[28] 1 of the best-known examples of taxa exhibiting facultative parthenogenesis are mayflies; presumably, this is the default reproductive mode of all species in this insect club.[29] Facultative parthenogenesis is believed to be a response to a lack of a viable male. A female may undergo facultative parthenogenesis if a male is absent from the habitat or if information technology is unable to produce viable offspring.
In aphids, a generation sexually conceived by a male and a female produces just females. The reason for this is the non-random segregation of the sex chromosomess 10 and O during spermatogenesis.[30]
Facultative parthenogenesis is often used to draw cases of spontaneous parthenogenesis in commonly sexual animals.[31] For example, many cases of spontaneous parthenogenesis in sharks, some snakes, Komodo dragons and a variety of domesticated birds were widely attributed to facultative parthenogenesis.[32] These cases are examples of spontaneous parthenogenesis.[28] [31] The occurrence of such asexually produced eggs in sexual animals tin be explained by a meiotic error, leading to eggs produced via automixis.[31] [33]
Obligate [edit]
Obligate parthenogenesis is the process in which organisms exclusively reproduce through asexual means.[34] Many species have been shown to transition to obligate parthenogenesis over evolutionary time. Well documented transitions to obligate parthenogenesis accept been found in numerous metazoan taxa, albeit through highly diverse mechanisms. These transitions often occur as a effect of inbreeding or mutation within large populations.[35] In that location are a number of documented species, specifically salamanders and geckos, that rely on obligate parthenogenesis as their major method of reproduction. As such, at that place are over 80 species of unisex reptiles (by and large lizards but including a unmarried ophidian species), amphibians and fishes in nature for which males are no longer a part of the reproductive process.[36] A female person will produce an ovum with a full set (two sets of genes) provided solely by the female parent. Thus, a male is not needed to provide sperm to fertilize the egg. This form of asexual reproduction is idea in some cases to be a serious threat to biodiversity for the subsequent lack of gene variation and potentially decreased fitness of the offspring.[34]
Some invertebrate species that feature (partial) sexual reproduction in their native range are plant to reproduce solely past parthenogenesis in areas to which they have been introduced.[37] [38] Relying solely on parthenogenetic reproduction has several advantages for an invasive species: it obviates the need for individuals in a very thin initial population to search for mates, and an exclusively female sex distribution allows a population to multiply and invade more rapidly, potentially up to twice as fast. Examples include several aphid species[37] and the willow sawfly, Nematus oligospilus, which is sexual in its native Holarctic habitat just parthenogenetic where information technology has been introduced into the Southern Hemisphere.[38]
Natural occurrence [edit]
Parthenogenesis is seen to occur naturally in aphids, Daphnia, rotifers, nematodes and another invertebrates, as well every bit in many plants. Among vertebrates, strict parthenogenesis is merely known to occur in lizards, snakes,[39] birds[forty] and sharks,[41] with fish, amphibians and reptiles exhibiting diverse forms of gynogenesis and hybridogenesis (an incomplete form of parthenogenesis).[42] The outset all-female (unisexual) reproduction in vertebrates was described in the fish Poecilia formosa in 1932.[43] Since then at least 50 species of unisexual vertebrate take been described, including at least xx fish, 25 lizards, a single ophidian species, frogs, and salamanders.[42] Other normally sexual species may occasionally reproduce parthenogenetically; the Komodo dragon and hammerhead and blacktip sharks are contempo additions to the known list of spontaneous parthenogenetic vertebrates. As with all types of asexual reproduction, at that place are both costs (low genetic diversity and therefore susceptibility to adverse mutations that might occur) and benefits (reproduction without the demand for a male) associated with parthenogenesis.
Parthenogenesis is distinct from artificial fauna cloning, a process where the new organism is necessarily genetically identical to the cell donor. In cloning, the nucleus of a diploid cell from a donor organism is inserted into an enucleated egg cell and the cell is and so stimulated to undergo continued mitosis, resulting in an organism that is genetically identical to the donor. Parthenogenesis is unlike, in that it originates from the genetic cloth contained within an egg jail cell and the new organism is not necessarily genetically identical to the parent.
Parthenogenesis may be achieved through an artificial procedure as described below under the word of mammals.
Oomycetes [edit]
Apomixis can apparently occur in Phytophthora,[44] an oomycete. Oospores from an experimental cross were germinated, and some of the progeny were genetically identical to one or other parent, implying that meiosis did not occur and the oospores developed past parthenogenesis.
Velvet worms [edit]
No males of Epiperipatus imthurni take been found, and specimens from Trinidad were shown to reproduce parthenogenetically. This species is the only known velvet worm to reproduce via parthenogenesis.[45]
Rotifers [edit]
In bdelloid rotifers, females reproduce exclusively by parthenogenesis (obligate parthenogenesis),[46] while in monogonont rotifers, females tin alternating between sexual and asexual reproduction (cyclical parthenogenesis). At to the lowest degree in one unremarkably cyclical parthenogenetic species obligate parthenogenesis tin can be inherited: a recessive allele leads to loss of sexual reproduction in homozygous offspring.[47]
Flatworms [edit]
At least 2 species in the genus Dugesia, flatworms in the Turbellaria sub-sectionalisation of the phylum Platyhelminthes, include polyploid individuals that reproduce past parthenogenesis.[48] This type of parthenogenesis requires mating, but the sperm does not contribute to the genetics of the offspring (the parthenogenesis is pseudogamous, alternatively referred to every bit gynogenetic). A complex cycle of matings between diploid sexual and polyploid parthenogenetic individuals produces new parthenogenetic lines.
Snails [edit]
Several species of parthenogenetic gastropods accept been studied, particularly with respect to their status every bit invasive species. Such species include the New Zealand mud snail (Potamopyrgus antipodarum),[49] the scarlet-rimmed melania (Melanoides tuberculata),[50] and the Quilted melania (Tarebia granifera).[51]
Insects [edit]
Parthenogenesis in insects can cover a wide range of mechanisms.[52] The offspring produced by parthenogenesis may be of both sexes, simply female (thelytoky, east.k. aphids and some hymenopterans[53]) or only male (arrhenotoky, east.thousand. most hymenopterans). Both true parthenogenesis and pseudogamy (gynogenesis or sperm-dependent parthenogenesis) are known to occur.[28] The egg cells, depending on the species may be produced without meiosis (apomictically) or by one of the several automictic mechanisms.
A related phenomenon, polyembryony is a process that produces multiple clonal offspring from a single egg jail cell. This is known in some hymenopteran parasitoids and in Strepsiptera.[52]
In automictic species the offspring can exist haploid or diploid. Diploids are produced by doubling or fusion of gametes later meiosis. Fusion is seen in the Phasmatodea, Hemiptera (Aleurodids and Coccidae), Diptera, and some Hymenoptera.[52]
In addition to these forms is hermaphroditism, where both the eggs and sperm are produced by the aforementioned individual, simply is not a type of parthenogenesis. This is seen in iii species of Icerya scale insects.[52]
Parasitic bacteria similar Wolbachia have been noted to induce automictic thelytoky in many insect species with haplodiploid systems. They also cause gamete duplication in unfertilized eggs causing them to develop into female person offspring.[52]

Amid species with the haplo-diploid sex-conclusion organization, such as hymenopterans (ants, bees and wasps) and thysanopterans (thrips), haploid males are produced from unfertilized eggs. Normally, eggs are laid simply by the queen, simply the unmated workers may also lay haploid, male person eggs either regularly (e.g. stingless bees) or under special circumstances. An example of non-viable parthenogenesis is common among domesticated dear bees. The queen bee is the merely fertile female in the hive; if she dies without the possibility of a viable replacement queen, it is not uncommon for the worker bees to lay eggs. This is a result of the lack of the queen'due south pheromones and the pheromones secreted by uncapped brood, which normally suppress ovarian evolution in workers. Worker bees are unable to mate, and the unfertilized eggs produce only drones (males), which tin can mate only with a queen. Thus, in a relatively brusk catamenia, all the worker bees die off, and the new drones follow if they have non been able to mate before the plummet of the colony. This behavior is believed to have evolved to let a doomed colony to produce drones which may mate with a virgin queen and thus preserve the colony'due south genetic progeny.
A few ants and bees are capable of producing diploid female offspring parthenogenetically. These include a dearest bee subspecies from South Africa, Apis mellifera capensis, where workers are capable of producing diploid eggs parthenogenetically, and replacing the queen if she dies; other examples include some species of small carpenter bee, (genus Ceratina). Many parasitic wasps are known to be parthenogenetic, sometimes due to infections past Wolbachia.
The workers in five[25] ant species and the queens in some ants are known to reproduce by parthenogenesis. In Cataglyphis cursor, a European formicine pismire, the queens and workers can produce new queens past parthenogenesis. The workers are produced sexually.[25]
In Cardinal and S American electric ants, Wasmannia auropunctata, queens produce more queens through automictic parthenogenesis with fundamental fusion. Sterile workers usually are produced from eggs fertilized by males. In some of the eggs fertilized by males, nevertheless, the fertilization tin cause the female genetic material to be ablated from the zygote. In this manner, males pass on but their genes to become fertile male offspring. This is the commencement recognized case of an animal species where both females and males tin can reproduce clonally resulting in a complete separation of male person and female person gene pools.[54] Equally a outcome, the males will only have fathers and the queens simply mothers, while the sterile workers are the only ones with both parents of both sexes.
These ants go both the benefits of both asexual and sexual reproduction[25] [54]—the daughters who can reproduce (the queens) have all of the mother's genes, while the sterile workers whose concrete forcefulness and disease resistance are important are produced sexually.
Other examples of insect parthenogenesis can be institute in gall-forming aphids (east.g., Pemphigus betae), where females reproduce parthenogenetically during the gall-forming phase of their life bike and in grass thrips. In the grass thrips genus Aptinothrips at that place have been, despite the very limited number of species in the genus, several transitions to asexuality.[55]
Crustaceans [edit]
Crustacean reproduction varies both beyond and within species. The water flea Daphnia pulex alternates between sexual and parthenogenetic reproduction.[56] Among the better-known large decapod crustaceans, some crayfish reproduce by parthenogenesis. "Marmorkrebs" are parthenogenetic crayfish that were discovered in the pet trade in the 1990s.[57] Offspring are genetically identical to the parent, indicating it reproduces past apomixis, i.east. parthenogenesis in which the eggs did non undergo meiosis.[58] Spinycheek crayfish (Orconectes limosus) tin can reproduce both sexually and by parthenogenesis.[59] The Louisiana ruddy swamp crayfish (Procambarus clarkii), which normally reproduces sexually, has also been suggested to reproduce past parthenogenesis,[threescore] although no individuals of this species accept been reared this way in the lab. Artemia parthenogenetica is a species or series of populations of parthenogenetic brine shrimps.[61]
Spiders [edit]
At least ii species of spiders in the family Oonopidae (goblin spiders), Heteroonops spinimanus and Triaeris stenaspis, are thought to be parthenogenetic, as no males have e'er been collected. Parthenogenetic reproduction has been demonstrated in the laboratory for T. stenaspis.[62]
Sharks [edit]
Parthenogenesis in sharks has been confirmed in at least iii species, the bonnethead,[41] the blacktip shark,[63] and the zebra shark,[64] and reported in others.
A bonnethead, a blazon of modest hammerhead shark, was found to accept produced a pup, built-in live on December 14, 2001, at Henry Doorly Zoo in Nebraska, in a tank containing three female person hammerheads, simply no males. The pup was idea to accept been conceived through parthenogenesis. The shark pup was apparently killed by a stingray inside days of birth.[65] The investigation of the nascence was conducted past the research team from Queen's University Belfast, Southeastern University in Florida, and Henry Doorly Zoo itself, and it was ended after DNA testing that the reproduction was parthenogenetic. The testing showed the female pup's DNA matched only 1 female who lived in the tank, and that no male DNA was present in the pup. The pup was non a twin or clone of her mother, but rather, contained only one-half of her female parent's DNA ("automictic parthenogenesis"). This type of reproduction had been seen before in bony fish, only never in cartilaginous fish such equally sharks, until this documentation.
In the aforementioned year, a female Atlantic blacktip shark in Virginia reproduced via parthenogenesis.[66] On Oct 10, 2008, scientists confirmed the second instance of a "virgin nascence" in a shark. The Journal of Fish Biology reported a study in which scientists said Dna testing proved that a pup carried by a female Atlantic blacktip shark in the Virginia Aquarium & Marine Science Middle contained no genetic material from a male.[63]
In 2002, two white-spotted bamboo sharks were built-in at the Belle Isle Aquarium in Detroit. They hatched 15 weeks after being laid. The births baffled experts every bit the female parent shared an aquarium with only one other shark, which was female. The female bamboo sharks had laid eggs in the past. This is not unexpected, every bit many animals will lay eggs fifty-fifty if there is not a male person to fertilize them. Normally, the eggs are assumed to be inviable and are discarded. This batch of eggs was left undisturbed by the curator equally he had heard about the previous birth in 2001 in Nebraska and wanted to observe whether they would hatch. Other possibilities had been considered for the birth of the Detroit bamboo sharks including thoughts that the sharks had been fertilized by a male and stored the sperm for a period of fourth dimension, likewise every bit the possibility that the Belle Isle bamboo shark is a hermaphrodite, harboring both male person and female sexual activity organs, and capable of fertilizing its ain eggs, merely that is non confirmed.[67]
In 2008, a Hungarian aquarium had another case of parthenogenesis later its lone female shark produced a pup without ever having come into contact with a male shark.
The repercussions of parthenogenesis in sharks, which fails to increase the genetic diversity of the offspring, is a affair of concern for shark experts, taking into consideration conservation management strategies for this species, particularly in areas where in that location may be a shortage of males due to fishing or ecology pressures. Although parthenogenesis may assist females who cannot discover mates, it does reduce genetic diversity.[ commendation needed ]
In 2011, recurring shark parthenogenesis over several years was demonstrated in a captive zebra shark, a blazon of rug shark.[64] [68] DNA genotyping demonstrated that individual zebra sharks can switch from sexual to parthenogenetic reproduction.[69]
Amphibians [edit]
Squamata [edit]

Komodo dragon, Varanus komodoensis, rarely reproduces offspring via parthenogenesis.
Most reptiles of the squamatan order (lizards and snakes) reproduce sexually, merely parthenogenesis has been observed to occur naturally in certain species of whiptails, some geckos, rock lizards,[5] [70] [71] Komodo dragons[72] and snakes.[73] Some of these like the mourning gecko Lepidodactylus lugubris, Indo-Pacific firm gecko Hemidactylus garnotii, the hybrid whiptails Cnemidophorus, Caucasian rock lizards Darevskia, and the brahminy blindsnake, Indotyphlops braminus are unisexual and obligately parthenogenetic. Other reptiles, such as the Komodo dragon, other monitor lizards,[74] and some species of boas,[ix] [26] [75] pythons,[24] [76] filesnakes,[77] [78] gartersnakes[79] and rattlesnakes[fourscore] [81] were previously considered equally cases of facultative parthenogenesis, simply are in fact cases of accidental parthenogenesis.[31]
In 2012, facultative parthenogenesis was reported in wild vertebrates for the beginning time past US researchers amongst captured pregnant copperhead and cottonmouth female pit-vipers.[82] The Komodo dragon, which normally reproduces sexually, has also been found able to reproduce asexually by parthenogenesis.[83] A case has been documented of a Komodo dragon reproducing via sexual reproduction later on a known parthenogenetic upshot,[84] highlighting that these cases of parthenogenesis are reproductive accidents, rather than adaptive, facultative parthenogenesis.[31]
Some reptile species use a ZW chromosome system, which produces either males (ZZ) or females (ZW). Until 2010, it was thought that the ZW chromosome arrangement used past reptiles was incapable of producing viable WW offspring, but a (ZW) female boa constrictor was discovered to have produced viable female offspring with WW chromosomes.[85]
Parthenogenesis has been studied extensively in the New United mexican states whiptail in the genus Aspidoscelis of which fifteen species reproduce exclusively by parthenogenesis. These lizards live in the dry and sometimes harsh climate of the southwestern United States and northern Mexico. All these asexual species appear to have arisen through the hybridization of two or 3 of the sexual species in the genus leading to polyploid individuals. The machinery past which the mixing of chromosomes from two or three species can pb to parthenogenetic reproduction is unknown. Recently, a hybrid parthenogenetic whiptail cadger was bred in the laboratory from a cross between an asexual and a sexual whiptail.[86] Because multiple hybridization events can occur, individual parthenogenetic whiptail species tin can consist of multiple independent asexual lineages. Inside lineages, there is very little genetic diversity, just unlike lineages may have quite different genotypes.
An interesting aspect to reproduction in these asexual lizards is that mating behaviors are still seen, although the populations are all female. 1 female plays the role played by the male in closely related species, and mounts the female that is about to lay eggs. This behaviour is due to the hormonal cycles of the females, which cause them to deport like males soon later on laying eggs, when levels of progesterone are high, and to take the female part in mating before laying eggs, when estrogen dominates. Lizards who act out the courtship ritual take greater fecundity than those kept in isolation, due to the increase in hormones that accompanies the mounting. Then, although the populations lack males, they notwithstanding crave sexual behavioral stimuli for maximum reproductive success.[87]
Some lizard parthenogens show a design of geographic parthenogenesis, occupying high mountain areas where their ancestral forms have an inferior competition power.[88] In Caucasian rock lizards of genus Darevskia, which have six parthenogenetic forms of hybrid origin[70] [71] [89] hybrid parthenogenetic grade D. "dahli" has a broader niche than either of its bisexual ancestors and its expansion throughout the Cardinal Bottom Caucasus caused decline of the ranges of both its maternal and paternal species.[90]
Birds [edit]
Parthenogenesis in birds is known mainly from studies of domesticated turkeys and chickens, although information technology has too been noted in the domestic pigeon.[40] In most cases the egg fails to develop normally or completely to hatching.[40] [91] The starting time description of parthenogenetic evolution in a passerine was demonstrated in captive zebra finches, although the dividing cells exhibited irregular nuclei and the eggs did non hatch.[twoscore]
Parthenogenesis in turkeys appears to upshot from a conversion of haploid cells to diploid;[91] about embryos produced in this way die early in evolution. Rarely, viable birds result from this procedure, and the rate at which this occurs in turkeys can be increased past selective convenance,[92] withal male turkeys produced from parthenogenesis showroom smaller testes and reduced fertility.[93]
In 2021, the San Diego Zoo reported that they had two unfertilized eggs from their California condor convenance programme hatch. This is the first known example of parthenogenesis in this species, as well equally one of the just known examples of parthenogenesis happening where males are nevertheless present.[8]
Mammals [edit]
There are no known cases of naturally occurring mammalian parthenogenesis in the wild. Parthenogenetic progeny of mammals would have two 10 chromosomes, and would therefore be genetically female person.
In 1936, Gregory Goodwin Pincus reported successfully inducing parthenogenesis in a rabbit.[94]
In April 2004, scientists at Tokyo University of Agriculture used parthenogenesis successfully to create a fatherless mouse. Using factor targeting, they were able to manipulate two imprinted loci H19/IGF2 and DLK1/MEG3 to produce bi-maternal mice at high frequency[95] and subsequently show that fatherless mice accept enhanced longevity.[96]
Induced parthenogenesis in mice and monkeys oft results in aberrant development. This is because mammals take imprinted genetic regions, where either the maternal or the paternal chromosome is inactivated in the offspring in order for evolution to keep normally. A mammal created past parthenogenesis would take double doses of maternally imprinted genes and lack paternally imprinted genes, leading to developmental abnormalities. It has been suggested[97] that defects in placental folding or interdigitation are i cause of swine parthenote abortive development. As a outcome, enquiry on human parthenogenesis is focused on the production of embryonic stem cells for use in medical treatment, not equally a reproductive strategy.
However, in 2022, researchers reported that they take achieved parthenogenesis in mice for feasible offspring born from unfertilized eggs, addressing the problems of genomic imprinting past "targeted Deoxyribonucleic acid methylation rewriting of seven imprinting command regions".[10]
Methods [edit]
Use of an electrical or chemic stimulus tin can produce the beginning of the process of parthenogenesis in the asexual development of viable offspring.[98]

Induction of parthenogenesis in swine. Parthenogenetic development of swine oocytes.[97] High metaphase promoting factor (MPF) activity causes mammalian oocytes to arrest at the metaphase II stage until fertilization by a sperm. The fertilization event causes intracellular calcium oscillations, and targeted degradation of cyclin B, a regulatory subunit of MPF, thus permitting the MII-arrested oocyte to proceed through meiosis. To initiate parthenogenesis of swine oocytes, diverse methods exist to induce an artificial activation that mimics sperm entry, such as calcium ionophore treatment, microinjection of calcium ions, or electrical stimulation. Treatment with cycloheximide, a non-specific poly peptide synthesis inhibitor, enhances parthenote development in swine presumably by continual inhibition of MPF/cyclin B.[99] As meiosis gain, extrusion of the second polar is blocked by exposure to cytochalasin B. This treatment results in a diploid (2 maternal genomes) parthenote. Parthenotes tin be surgically transferred to a recipient oviduct for further development, simply volition succumb past developmental failure later ≈thirty days of gestation. The swine parthenote placentae often appears hypo-vascular and is approximately fifty% smaller than biparental offspring placentae: come across free image (Figure ane) in linked reference.[97]
During oocyte evolution, high metaphase promoting cistron (MPF) activity causes mammalian oocytes to arrest at the metaphase II stage until fertilization by a sperm. The fertilization event causes intracellular calcium oscillations, and targeted deposition of cyclin B, a regulatory subunit of MPF, thus permitting the MII-arrested oocyte to keep through meiosis.
To initiate parthenogenesis of swine oocytes, various methods exist to induce an artificial activation that mimics sperm entry, such as calcium ionophore treatment, microinjection of calcium ions, or electrical stimulation. Treatment with cycloheximide, a not-specific protein synthesis inhibitor, enhances parthenote development in swine presumably by continual inhibition of MPF/cyclin B.[99] As meiosis proceeds, extrusion of the 2nd polar is blocked by exposure to cytochalasin B. This handling results in a diploid (ii maternal genomes) parthenote[97] Parthenotes can exist surgically transferred to a recipient oviduct for farther evolution, but will succumb to developmental failure after ≈xxx days of gestation. The swine parthenote placentae often appears hypo-vascular: come across gratis image (Figure 1) in linked reference.[97]
Humans [edit]
Reports of human parthenogenesis have famously existed since aboriginal times, featuring prominently in Christianity and various other religions. More recently, Helen Spurway, a geneticist specializing in the reproductive biology of the guppy (Lebistes reticulatus), claimed in 1955 that parthenogenesis, which occurs in the guppy in nature, may as well occur (though very rarely) in the human being species, leading to so-called "virgin births". This created some sensation amongst her colleagues and the lay public alike.[100] Sometimes an embryo may brainstorm to carve up without fertilisation, only it cannot fully develop on its own; then while it may create some skin and nerve cells, information technology cannot create others (such as skeletal muscle) and becomes a type of benign tumor called an ovarian teratoma.[101] Spontaneous ovarian activation is not rare and has been known about since the 19th century. Some teratomas tin can even get primitive fetuses (fetiform teratoma) with imperfect heads, limbs and other structures, only are non-viable.
In 1995, there was a reported case of fractional human parthenogenesis; a boy was plant to have some of his cells (such equally white blood cells) to be lacking in any genetic content from his father. Scientists believe that an unfertilised egg began to self-divide only then had some (but non all) of its cells fertilised by a sperm jail cell; this must have happened early on in development, as cocky-activated eggs rapidly lose their ability to be fertilised. The unfertilised cells eventually duplicated their DNA, boosting their chromosomes to 46. When the unfertilised cells striking a developmental block, the fertilised cells took over and developed that tissue. The male child had asymmetrical facial features and learning difficulties simply was otherwise healthy. This would make him a parthenogenetic chimera (a kid with two cell lineages in his body).[102] While over a dozen similar cases accept been reported since then (unremarkably discovered subsequently the patient demonstrated clinical abnormalities), at that place have been no scientifically confirmed reports of a non-chimeric, clinically healthy human being parthenote (i.e. produced from a unmarried, parthenogenetic-activated oocyte).[101]
On June 26, 2007, the International Stalk Cell Corporation (ISCC), a California-based stem jail cell enquiry company, announced that their pb scientist, Dr. Elena Revazova, and her research team were the kickoff to intentionally create human stem cells from unfertilized human eggs using parthenogenesis. The process may offering a way for creating stem cells that are genetically matched to a particular female person for the treatment of degenerative diseases that might impact her. In December 2007, Dr. Revazova and ISCC published an article[103] illustrating a breakthrough in the apply of parthenogenesis to produce homo stalk cells that are homozygous in the HLA region of DNA. These stem cells are called HLA homozygous parthenogenetic man stalk cells (hpSC-Hhom) and have unique characteristics that would permit derivatives of these cells to be implanted into millions of people without immune rejection.[104] With proper selection of oocyte donors co-ordinate to HLA haplotype, it is possible to generate a bank of prison cell lines whose tissue derivatives, collectively, could be MHC-matched with a meaning number of individuals within the homo population.
On August ii, 2007, after an contained investigation, it was revealed that discredited South Korean scientist Hwang Woo-Suk unknowingly produced the starting time homo embryos resulting from parthenogenesis. Initially, Hwang claimed he and his team had extracted stalk cells from cloned human embryos, a result later found to be fabricated. Further examination of the chromosomes of these cells bear witness indicators of parthenogenesis in those extracted stalk cells, similar to those found in the mice created by Tokyo scientists in 2004. Although Hwang deceived the world about being the get-go to create artificially cloned human embryos, he contributed a major breakthrough to stem cell research by creating human embryos using parthenogenesis.[105] The truth was discovered in 2007, long after the embryos were created by him and his team in Feb 2004. This made Hwang the first, unknowingly, to successfully perform the process of parthenogenesis to create a human being embryo and, ultimately, a human being parthenogenetic stem prison cell line.
A United states study conducted between 1995 and 2009 establish that out of vii,870 young women who participated, 45 (0.5%) reported that they had had a virgin pregnancy and resulting birth. The alleged virgin mothers were found to be relatively unlikely to know how to use a prophylactic, and 67.8% said they would endorse prophylactic use. Additionally, they were relatively likely to have pledged chastity and to accept had less communication with their parents about sex and birth control.[106]
Similar phenomena [edit]
Gynogenesis [edit]
A form of asexual reproduction related to parthenogenesis is gynogenesis. Here, offspring are produced past the same mechanism as in parthenogenesis, just with the requirement that the egg merely be stimulated past the presence of sperm in order to develop. However, the sperm prison cell does not contribute any genetic material to the offspring. Since gynogenetic species are all female, activation of their eggs requires mating with males of a closely related species for the needed stimulus. Some salamanders of the genus Ambystoma are gynogenetic and appear to take been and so for over a million years. It is believed[ by whom? ] that the success of those salamanders may be due to rare fertilization of eggs by males, introducing new material to the factor pool, which may result from perhaps simply 1 mating out of a million. In addition, the amazon molly is known to reproduce past gynogenesis.[107]
Hybridogenesis [edit]
Hybridogenesis is a mode of reproduction of hybrids. Hybridogenetic hybrids (for case AB genome), ordinarily females, during gametogenesis exclude one of parental genomes (A) and produce gametes with unrecombined[108] genome of second parental species (B), instead of containing mixed recombined parental genomes. First genome (A) is restored by fertilization of these gametes with gametes from the first species (AA, sexual host,[108] ordinarily male).[108] [109] [110]
And then hybridogenesis is not completely asexual, but instead hemiclonal: half of genome is passed to the next generation clonally, unrecombined, intact (B), other half sexually, recombined (A).[108] [111]
This procedure continues, and then that each generation is half (or hemi-) clonal on the female parent's side and has half new genetic fabric from the male parent's side.
This course of reproduction is seen in some live-bearing fish of the genus Poeciliopsis [109] [112] every bit well every bit in some of the Pelophylax spp. ("green frogs" or "waterfrogs"):
- P. kl. esculentus (edible frog): P. lessonae × P. ridibundus,[108] [113] [114]
- P. kl. grafi (Graf'southward hybrid frog): P. perezi × P. ridibundus [108]
- P. kl. hispanicus (Italian edible frog) – unknown origin: P. bergeri × P. ridibundus or P. kl. esculentus [108]
and perhaps in P. demarchii.
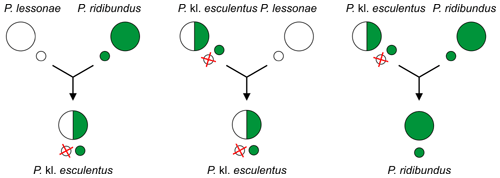
Example crosses between pool frog (Pelophylax lessonae), marsh frog (P. ridibundus) and their hybrid – edible frog (P. kl. esculentus). First i is the primary hybridisation generating hybrid, second 1 is most widespread type of hybridogenesis.[108] [115]
Other examples where hybridogenesis is at to the lowest degree one of modes of reproduction include i.e.
- Iberian minnow Tropidophoxinellus alburnoides (Squalius pyrenaicus × hypothetical ancestor related with Anaecypris hispanica)[116]
- spined loaches Cobitis hankugensis × C. longicorpus [117]
- Bacillus stick insects B. rossius × Bacillus grandii benazzii[118]
See also [edit]
- Charles Bonnet – Genevan botanist (1720–1793) – conducted experiments that established what is now termed parthenogenesis in aphids
- Jan Dzierżon – Shine apiarist and a pioneer of parthenogenesis amidst bees
- Jacques Loeb – caused the eggs of sea urchins to begin embryonic evolution without sperm
- Miraculous births
- Parthenocarpy – Production of fruit without fertilisation which makes the fruit seedless – plants with seedless fruit
References [edit]
- ^ "parthenogenesis". Merriam-Webster Lexicon.
- ^ "parthenogenesis - definition of parthenogenesis in English from the Oxford dictionary". OxfordDictionaries.com. Retrieved 2016-01-twenty .
- ^ Liddell, Scott, Jones. γένεσις A.II, A Greek-English Lexicon, Oxford: Clarendon Press, 1940. q.v..
- ^ "Female Sharks Tin can Reproduce Alone, Researchers Notice", Washington Post, Wednesday, May 23, 2007; p. A02
- ^ a b Halliday, Tim R. (1986). Kraig Adler (ed.). Reptiles & Amphibians. Torstar Books. p. 101. ISBN978-0-920269-81-7.
- ^ Walker, Brian (November 11, 2010). "Scientists discover unknown lizard species at lunch buffet". CNN . Retrieved 2010-11-11 .
- ^ Cruel, Thomas F. (September 12, 2005). "A Guide to the Recognition of Parthenogenesis in Incubated Turkey Eggs". Oregon State University . Retrieved 2006-10-xi .
- ^ a b Ryder OA, Thomas S, Judson JM, Romanov MN, Dandekar Southward, Papp JC, Sidak-Loftis LC, Walker K, Stalis IH, Mace M, Steiner CC, Chemnick LG (Oct 28, 2021). "Facultative Parthenogenesis in California Condors". Journal of Heredity. 112 (vii): 569–574. doi:ten.1093/jhered/esab052. PMID 34718632. Retrieved 2021-10-31 .
- ^ a b Booth, W.; Johnson, D. H.; Moore, S.; Schal, C.; Vargo, E. L. (2010). "Evidence for viable, not-clonal simply fatherless Boa constrictors". Biology Letters. 7 (two): 253–256. doi:10.1098/rsbl.2010.0793. PMC3061174. PMID 21047849.
- ^ a b Wei Y, Yang CR, Zhao ZA (March 7, 2022). "Viable offspring derived from single unfertilized mammalian oocytes". PNAS. 119 (12). doi:10.1073/pnas.2115248119. PMID 35254875. Retrieved 2022-03-11 .
- ^ Smith, John Maynard (1978). The Development of Sexual practice. Loving cup Archive. p. 42. ISBN978-0-521-21887-0.
- ^ Scott, Thomas (1996). Curtailed encyclopedia biology . Walter de Gruyter. ISBN978-3-eleven-010661-9.
- ^ Poinar, George O Jr; Trevor A Jackson; Nigel Fifty Bell; Mohd B-asri Wahid (July 2002). "Elaeolenchus parthenonema due north. g., n. sp. (Nematoda: Sphaerularioidea: Anandranematidae n. fam.) parasitic in the palm-pollinating weevil Elaeidobius kamerunicus Faust, with a phylogenetic synopsis of the Sphaerularioidea Lubbock, 1861". Systematic Parasitology. 52 (iii): 219–225. doi:10.1023/A:1015741820235. ISSN 0165-5752. PMID 12075153. S2CID 6405965.
- ^ White, Michael J.D. (1984). "Chromosomal Mechanisms in Animal Reproduction". Bolletino di Zoologia. 51 (1–2): 1–23. doi:10.1080/11250008409439455. ISSN 0373-4137.
- ^ Pujade-Villar, Juli; D. Bellido; G. Segu; George Melika (2001). "Current country of knowledge of heterogony in Cynipidae (Hymenoptera, Cynipoidea)". Sessio Conjunta DEntomologia ICHNSCL. 11 (1999): 87–107.
- ^ Bernstein, H; Hopf, FA; Michod, RE (1987). The molecular basis of the development of sex. Adv Genet. Advances in Genetics. Vol. 24. pp. 323–370. doi:x.1016/s0065-2660(08)60012-7. ISBN978-0-12-017624-3. PMID 3324702.
- ^ Gavrilov, I.A.; Kuznetsova, Five.Yard. (2007). "On some terms used in the cytogenetics and reproductive biology of scale insects (Homoptera: Coccinea)" (PDF). Comparative Cytogenetics. 1 (two): 169–174. ISSN 1993-078X.
- ^ Engelstädter, Jan (2017). "Asexual only Not Clonal: Evolutionary Processes in Automictic Populations | Genetics". Genetics. 206 (2): 993–1009. doi:10.1534/genetics.116.196873. PMC5499200. PMID 28381586.
- ^ a b Mogie, Michael (1986). "Automixis: its distribution and condition". Biological Journal of the Linnean Lodge. 28 (3): 321–329. doi:x.1111/j.1095-8312.1986.tb01761.x.
- ^ Zakharov, I. A. (April 2005). "Intratetrad mating and its genetic and evolutionary consequences". Russian Periodical of Genetics. 41 (4): 402–411. doi:10.1007/s11177-005-0103-z. ISSN 1022-7954. PMID 15909911. S2CID 21542999.
- ^ a b c Cosín, Darío J. Díaz, Marta Novo, and Rosa Fernández. "Reproduction of Earthworms: Sexual Selection and Parthenogenesis." In Biological science of Earthworms, edited past Ayten Karaca, 24:69–86. Berlin, Heidelberg: Springer Berlin Heidelberg, 2011. https://doi.org/10.1007%2F978-three-642-14636-7_5.
- ^ a b Cuellar, Orlando (February 1, 1971). "Reproduction and the mechanism of meiotic restitution in the parthenogenetic lizard Cnemidophorus uniparens". Journal of Morphology. 133 (2): 139–165. doi:ten.1002/jmor.1051330203. ISSN 1097-4687. PMID 5542237. S2CID 19729047.
- ^ a b c Lokki, Juhani; Esko Suomalainen; Anssi Saura; Pekka Lankinen (March ane, 1975). "Genetic Polymorphism and Development in Parthenogenetic Animals. Ii. Diploid and Polyploid Solenobia Triquetrella (lepidoptera: Psychidae)". Genetics. 79 (3): 513–525. doi:ten.1093/genetics/79.three.513. PMC1213290. PMID 1126629. Retrieved 2011-12-20 .
- ^ a b c d Groot, T 5 M; E Bruins; J A J Breeuwer (February 28, 2003). "Molecular genetic testify for parthenogenesis in the Burmese python, Python molars bivittatus". Heredity. 90 (2): 130–135. CiteSeerXten.ane.i.578.4368. doi:x.1038/sj.hdy.6800210. ISSN 0018-067X. PMID 12634818. S2CID 2972822.
- ^ a b c d Pearcy, M.; Aron, S; Doums, C; Keller, Fifty (2004). "Provisional Utilise of Sex activity and Parthenogenesis for Worker and Queen Production in Ants". Science. 306 (5702): 1780–1783. Bibcode:2004Sci...306.1780P. doi:10.1126/scientific discipline.1105453. PMID 15576621. S2CID 37558595.
- ^ a b c d due east Booth, Warren; Larry Million; R. Graham Reynolds; Gordon Thousand. Burghardt; Edward 50. Vargo; Coby Schal; Athanasia C. Tzika; Gordon W. Schuett (Dec 2011). "Consecutive Virgin Births in the New World Boid Snake, the Colombian Rainbow Boa, Epicrates maurus". Journal of Heredity. 102 (6): 759–763. doi:10.1093/jhered/esr080. PMID 21868391.
- ^ Hales, Dinah F.; Alex C. C. Wilson; Mathew A. Sloane; Jean-Christophe Simon; Jean-François Legallic; Paul Sunnucks (2002). "Lack of Detectable Genetic Recombination on the Ten Chromosome During the Parthenogenetic Production of Female person and Male person Aphids". Genetics Inquiry. 79 (3): 203–209. doi:10.1017/S0016672302005657. PMID 12220127.
- ^ a b c d Bell, G. (1982). The Masterpiece of Nature: The Development and Genetics of Sexuality, University of California Press, Berkeley, pp. i–635 (encounter p. 295). ISBN 978-0-520-04583-5
- ^ Funk, David H.; Sweeney, Bernard W.; Jackson, John Grand. (2010). "Why stream mayflies tin reproduce without males but remain bisexual: A case of lost genetic variation". Periodical of the North American Benthological Gild. 29 (4): 1258–1266. doi:10.1899/ten-015.1. S2CID 86088826.
- ^ Schwartz, Hermann (1932). "Der Chromosomenzyklus von Tetraneura ulmi de Geer". Zeitschrift für Zellforschung und Mikroskopische Anatomie. 15 (4): 645–687. doi:ten.1007/BF00585855. S2CID 43030757.
- ^ a b c d e van der Kooi, C.J.; Schwander, T. (2015). "Parthenogenesis: nativity of a new lineage or reproductive blow?" (PDF). Current Biology. 25 (15): R659–R661. doi:x.1016/j.cub.2015.06.055. PMID 26241141.
- ^ Lampert, K.P. (2008). "Facultative Parthenogenesis in Vertebrates: Reproductive Error or Take a chance?". Sexual Development. 2 (6): 290–301. doi:10.1159/000195678. PMID 19276631. S2CID 9137566.
- ^ Suomalainen E. et al. (1987). Cytology and Evolution in Parthenogenesis, Boca Raton, CRC Press
- ^ a b Stelzer C-P, Schmidt J, Wiedlroither A, Riss S (2010) Loss of Sexual Reproduction and Dwarfing in a Small Metazoan. PLoS.
- ^ Scheuerl, Thomas., et al. "Phenotypic of an Allele Causing Obligate Parthenogenesis." (2011). Journal of Heredity 2011:102(four):409–415. Spider web. 23 October. 2012
- ^ Booth, W.; Smith, C. F.; Eskridge, P. H.; Hoss, S. K.; Mendelson, J. R.; Schuett, G. Due west. (2012). "Facultative parthenogenesis discovered in wild vertebrates". Biology Letters. viii (six): 983–985. doi:10.1098/rsbl.2012.0666. PMC3497136. PMID 22977071.
- ^ a b Vorburger, Christoph (2003). "Environmentally related patterns of reproductive modes in the aphid Myzus persicae and the predominance of two 'superclones' in Victoria, Commonwealth of australia". Molecular Ecology. 12 (12): 3493–3504. doi:10.1046/j.1365-294X.2003.01998.x. PMID 14629364. S2CID 32192796.
- ^ a b Caron, V.; Norgate, M.; Ede, F.J.; Nyman, T. (2013). "Novel microsatellite DNA markers indicate strict parthenogenesis and few genotypes in the invasive willow sawfly Nematus oligospilus" (PDF). Bulletin of Entomological Enquiry. 103 (one): 74–88. doi:10.1017/S0007485312000429. PMID 22929915. S2CID 25210471.
- ^ Price, A. H. (1992). Comparative behavior in lizards of the genus Cnemidophorus (Teiidae), with comments on the evolution of parthenogenesis in reptiles. Copeia, 323–331.
- ^ a b c d Schut, E.; Hemmings, N.; Birkhead, T. R. (2008). "Parthenogenesis in a passerine bird, the Zebra Finch Taeniopygia guttata". Ibis. 150 (i): 197–199. doi:10.1111/j.1474-919x.2007.00755.x.
- ^ a b Chapman, Demian D.; Shivji, Mahmood Due south.; Louis, Ed; Sommer, Julie; Fletcher, Hugh; Prodöhl, Paulo A. (2007). "Virgin birth in a hammerhead shark". Biological science Messages. 3 (4): 425–427. doi:x.1098/rsbl.2007.0189. PMC2390672. PMID 17519185.
- ^ a b Vrijenhoek, R.C., R.Thousand. Dawley, C.J. Cole, and J.P. Bogart. 1989. A listing of the known unisexual vertebrates, pp. 19–23 in: Evolution and Ecology of Unisexual Vertebrates. R.M. Dawley and J.P. Bogart (eds.) Bulletin 466, New York Land Museum, Albany
- ^ Hubbs, C. L.; Hubbs, L. C. (1932). "Apparent parthenogenesis in nature, in a form of fish of hybrid origin". Science. 76 (1983): 628–630. Bibcode:1932Sci....76..628H. doi:10.1126/science.76.1983.628. PMID 17730035.
- ^ Hurtado-Gonzales, O. P.; Lamour, K. H. (2009). "Evidence for inbreeding and apomixis in shut crosses ofPhytophthora capsici". Plant Pathology. 58 (4): 715–722. doi:10.1111/j.1365-3059.2009.02059.x.
- ^ Read, V. M. St. J. (July 1988). "The Onychophora of Trinidad, Tobago, and the Lesser Antilles". Zoological Journal of the Linnean Gild. 93 (three): 225–257. doi:x.1111/j.1096-3642.1988.tb01362.x.
- ^ "Bdelloids: No sex for over 40 1000000 years.". TheFreeLibrary. ScienceNews. Retrieved 30 April 2011.
- ^ Stelzer, C.-P.; Schmidt, J.; Wiedlroither, A.; Riss, South. (2010). "Loss of Sexual Reproduction and Dwarfing in a Pocket-size Metazoan". PLOS Ane. 5 (9): e12854. Bibcode:2010PLoSO...512854S. doi:10.1371/periodical.pone.0012854. PMC2942836. PMID 20862222.
- ^ Lentati, G. Benazzi (1966). "Amphimixis and pseudogamy in fresh-water triclads: Experimental reconstitution of polyploid pseudogamic biotypes". Chromosoma. 20: 1–14. doi:10.1007/BF00331894. S2CID 21654518.
- ^ Wallace, C. (1992). "arthenogenesis, sex and chromosomes in Potamopyrgus". Periodical of Molluscan Studies. 58 (2): 93–107. doi:10.1093/mollus/58.2.93.
- ^ Ben-Ami, F.; Heller, J. (2005). "Spatial and temporal patterns of parthenogenesis and parasitism in the freshwater snail Melanoides tuberculata". Periodical of Evolutionary Biology. 18 (one): 138–146. doi:x.1111/j.1420-9101.2004.00791.10. PMID 15669970. S2CID 10422561.
- ^ Miranda, Nelson A. F.; Perissinotto, Renzo; Appleton, Christopher C.; Lalueza-Play a joke on, Carles (2011). "Population Structure of an Invasive Parthenogenetic Gastropod in Coastal Lakes and Estuaries of Northern KwaZulu-Natal, South Africa". PLOS One. 6 (8): e24337. Bibcode:2011PLoSO...624337M. doi:10.1371/journal.pone.0024337. PMC3164166. PMID 21904629.
- ^ a b c d eastward Kirkendall, 50. R. & Normark, B. (2003) Parthenogenesis in Encyclopaedia of Insects (Vincent H. Resh and R. T. Carde, Eds.) Bookish Press. pp. 851–856
- ^ Copeland, Claudia S.; Hoy, Marjorie A.; Jeyaprakash, Ayyamperumal; Aluja, Martin; Ramirez-Romero, Ricardo; Sivinski, John One thousand. (September i, 2010). "Genetic Characteristics of Bisexual and Female-Just Populations of Odontosema anastrephae (Hymenoptera: Figitidae)". Florida Entomologist. 93 (3): 437–443. doi:ten.1653/024.093.0318.
- ^ a b Fournier, Denis; Estoup, Arnaud; Orivel, Jérôme; Foucaud, Julien; Jourdan, Hervé; Le Breton, Julien Le; Keller, Laurent (2005). "Clonal reproduction past males and females in the picayune fire ant" (PDF). Nature. 435 (7046): 1230–1234. Bibcode:2005Natur.435.1230F. doi:ten.1038/nature03705. PMID 15988525. S2CID 1188960.
- ^ CJ van der Kooi & T Schwander (2014) Evolution of asexuality via different mechanisms in grass thrips (Thysanoptera: Aptinothrips) Evolution 86:1883–1893
- ^ Eads, Brian D; Colbourne, John Grand; Bohuski, Elizabeth; Andrews, Justen (2007). "Profiling sex-biased factor expression during parthenogenetic reproduction in Daphnia pulex". BMC Genomics. viii: 464. doi:10.1186/1471-2164-viii-464. PMC2245944. PMID 18088424.
- ^ Scholtz, Gerhard; Braband, Anke; Tolley, Laura; Reimann, André; Mittmann, Beate; Lukhaup, Chris; Steuerwald, Frank; Vogt, GüNter (2003). "Ecology: Parthenogenesis in an outsider crayfish". Nature. 421 (6925): 806. Bibcode:2003Natur.421..806S. doi:x.1038/421806a. PMID 12594502. S2CID 84740187.
- ^ Martin, Peer; Kohlmann, Klaus; Scholtz, Gerhard (2007). "The parthenogenetic Marmorkrebs (marbled crayfish) produces genetically compatible offspring". Naturwissenschaften. 94 (10): 843–846. Bibcode:2007NW.....94..843M. doi:x.1007/s00114-007-0260-0. PMID 17541537. S2CID 21568188.
- ^ Buřič, Miloš; Hulák, Martin; Kouba, Antonín; Petrusek, Adam; Kozák, Pavel; Etges, William J. (May 31, 2011). Etges, William J (ed.). "A Successful Crayfish Invader Is Capable of Facultative Parthenogenesis: A Novel Reproductive Fashion in Decapod Crustaceans". PLOS ONE. 6 (5): e20281. Bibcode:2011PLoSO...620281B. doi:ten.1371/journal.pone.0020281. PMC3105005. PMID 21655282.
- ^ Yue GH, Wang GL, Zhu BQ, Wang CM, Zhu ZY, Lo LC (2008). "Discovery of four natural clones in a crayfish species Procambarus clarkii". International Journal of Biological Sciences. 4 (5): 279–282. doi:10.7150/ijbs.4.279. PMC2532795. PMID 18781225.
- ^ Muñoz, Joaquín; Gómez, Africa; Light-green, Andy J.; Figuerola, Jordi; Amat, Francisco; Rico, Ciro; Moreau, Corrie S. (August iv, 2010). "Evolutionary Origin and Phylogeography of the Diploid Obligate Parthenogen Artemia parthenogenetica (Branchiopoda: Anostraca)". PLOS ONE. 5 (8): e11932. Bibcode:2010PLoSO...511932M. doi:10.1371/periodical.pone.0011932. PMC2915914. PMID 20694140.
- ^ Korenko, Stanislav; Šmerda, Jakub & Pekár, Stano (2009). "Life-history of the parthenogenetic oonopid spider, Triaeris stenaspis (Araneae: Oonopidae)". European Periodical of Entomology. 106 (2): 217–223. doi:10.14411/eje.2009.028 . Retrieved 2016-04-xxx .
- ^ a b Chapman, D. D.; Firchau, B.; Shivji, Thousand. S. (2008). "Parthenogenesis in a large-bodied requiem shark, the blacktip". Journal of Fish Biological science. 73 (6): 1473–1477. doi:10.1111/j.1095-8649.2008.02018.x.
- ^ a b Robinson, D. P.; Baverstock, W.; Al-Jaru, A.; Hyland, K.; Khazanehdari, Thou. A. (2011). "Annually recurring parthenogenesis in a zebra shark Stegostoma fasciatum". Periodical of Fish Biological science. 79 (5): 1376–1382. doi:10.1111/j.1095-8649.2011.03110.x. PMID 22026614.
- ^ "Convict shark had 'virgin nascency'". BBC News. May 23, 2007. Retrieved 2008-12-23 .
- ^ "'Virgin nativity' for aquarium shark". Metro.co.uk. Oct x, 2008. Retrieved 2008-10-ten .
- ^ National Geographic, (2002). "Shark gives virgin birth in Detroit". Retrieved April. 17, 2010, from Nationalgeographic.com Spider web site:
- ^ "Starting time Virgin Nascency of Zebra Shark in Dubai". Sharkyear.com.
- ^ Dudgeon, Christine L.; Coulton, Laura; Bone, Ren; Ovenden, Jennifer R.; Thomas, Severine (Jan 16, 2017). "Switch from sexual to parthenogenetic reproduction in a zebra shark". Scientific Reports. 7: 40537. Bibcode:2017NatSR...740537D. doi:10.1038/srep40537. ISSN 2045-2322. PMC5238396. PMID 28091617.
- ^ a b Darevskii IS. 1967. Rock lizards of the Caucasus: systematics, environmental and phylogenesis of the polymorphic groups of Caucasian rock lizards of the subgenus Archaeolacerta. Nauka: Saint petersburg [in Russian: English translation published by the Indian National Scientific Documentation Centre, New Delhi, 1978].
- ^ a b Tarkhnishvili DN (2012) Evolutionary History, Habitats, Diversification, and Speciation in Caucasian Rock Lizards. In: Advances in Zoology Research, Book two (ed. Jenkins OP), Nova Science Publishers, Hauppauge (NY), pp. 79–120
- ^ Watts, P. C.; Buley, Thou. R.; Sanderson, S.; Boardman, W.; Ciofi, C.; Gibson, R. (2006). "Parthenogenesis in Komodo dragons". Nature. 444 (7122): 1021–1022. Bibcode:2006Natur.444.1021W. doi:10.1038/4441021a. PMID 17183308. S2CID 4311088.
- ^ Cocky-impregnated snake in Missouri has another 'virgin birth', UPI, 21 September 2015. Retrieved 3 October 2015.
- ^ Wiechmann, R. (2012). "Observations of parthenogenesis in monitor lizards" (PDF). Biawak. 6 (1): xi–21.
- ^ Kinney, M.Due east.; Wack, R.F.; Grahn, R.A.; Lyons, Fifty. (2013). "Parthenogenesis in a Brazilian rainbow boa (Epicrates cenchria cenchria)". Zoo Biology. 32 (2): 172–176. doi:10.1002/zoo.21050. PMID 23086743.
- ^ Shepherd, Kyle (Dec 18, 2014). "A Virgin Snake Nascence".
- ^ Magnusson, W.Eastward. (1979). "Product of an embryo by an Acrochordus javanicus isolated for seven years". Copeia. 1979 (4): 744–745. doi:10.2307/1443886. JSTOR 1443886.
- ^ Dubach, J.; Sajewicz, A.; Pawley, R. (1997). "Parthenogenesis in the Arafura filesnake (Acrochordus arafurae)". Herpetological Natural History. 5 (1): 11–18.
- ^ Reynolds, R.G.; Booth, Due west.; Schuett, Thousand.West.; Fitzpatrick, B.M.; Burghardt, Yard.M. (2012). "Successive virgin births of viable male progeny in the checkered gartersnake, Thamnophis marcianus". Biological Periodical of the Linnean Society. 107 (3): 566–572. doi:10.1111/j.1095-8312.2012.01954.x.
- ^ Schuett, G.Due west.; Fernandez, P.J.; Gergits, Westward.F.; Casna, N.J..; Chiszar, D.; Smith, H.M.; Mitton, J.B.; Mackessy, S.P.; Odum, R.A.; Demlong, M.J. (1997). "Production of offspring in the absence of males: Testify for facultative parthenogenesis in bisexual snakes". Herpetological Natural History. 5 (ane): 1–10.
- ^ Schuett, G.Due west.; Fernandez, P.J.; Chiszar, D.; Smith, H.1000. (1998). "Fatherless Sons: A new type of parthenogenesis in snakes". Fauna. i (3): 20–25.
- ^ "Virgin births discovered in wild snakes". September 12, 2012. Retrieved 2012-09-12 .
- ^ "No sex activity please, nosotros're lizards", Roger Highfield, Daily Telegraph, 21 December 2006
- ^ Virgin birth of dragons, The Hindu, 25 Jan 2007. Retrieved 3 February 2007.
- ^ Walker, Matt (November 3, 2010). "Snake has unique 'virgin birth'". BBC News.
- ^ Lutes, Aracely A.; Diana P. Baumann; William B. Neaves; Peter Baumann (June xiv, 2011). "Laboratory synthesis of an independently reproducing vertebrate species". Proceedings of the National Academy of Sciences. 108 (24): 9910–9915. Bibcode:2011PNAS..108.9910L. doi:10.1073/pnas.1102811108. PMC3116429. PMID 21543715.
- ^ Crews, D.; Grassman, M.; Lindzey, J. (1986). "Behavioral Facilitation of Reproduction in Sexual and Unisexual Whiptail Lizards". Proceedings of the National Academy of Sciences. 83 (24): 9547–9550. Bibcode:1986PNAS...83.9547C. doi:10.1073/pnas.83.24.9547. PMC387177. PMID 3467325.
- ^ Vrijenhoek RC, Parker ED. 2009. Geographical parthenogenesis: general purpose genotypes and frozen niche variation. In: Schön I, Martens Yard, Van Dijk P, eds. Lost sexual activity. Berlin: Springer Publications, 99–131
- ^ Murphy, RW; Darevsky, IS; MacCulloch, RD; Fu, J; Kupriyanova, LA; Upton, DE; Danielyan, F. (1997). "Former age, multiple formations or genetic plasticity? Clonal diverseness in a parthenogenetic Caucasian rock lizard, Lacerta dahli". Genetica. 101 (two): 125–130. doi:10.1023/A:1018392603062. PMID 16220367. S2CID 11145792.
- ^ Tarkhnishvili, D; Gavashelishvili, A; Avaliani, A; Murtskhvaladze, M; Mumladze, 50 (2010). "Unisexual rock lizard might be outcompeting its bisexual progenitors in the Caucasus". Biological Journal of the Linnean Lodge. 101 (2): 447–460. doi:10.1111/j.1095-8312.2010.01498.x.
- ^ a b Mittwoch, U (1978). "Parthenogenesis". Periodical of Medical Genetics. 15 (iii): 165–181. doi:ten.1136/jmg.fifteen.3.165. PMC1013674. PMID 353283.
- ^ Nestor, Karl (2009). "Parthenogenesis in turkeys". The Tremendous Turkey. The Ohio State University. Archived from the original on 2010-07-14.
- ^ Sarvella, P (1974). "Testes structure in normal and parthenogenetic turkeys". The Journal of Heredity. 65 (5): 287–290. doi:10.1093/oxfordjournals.jhered.a108530. PMID 4373503.
- ^ Pincus, Gregory (2018). The eggs of mammals. New York: The Macmillan Visitor – via Net Archive.
- ^ Kawahara, Manabu; Wu, Qiong; Takahashi, Nozomi; Morita, Shinnosuke; Yamada, Kaori; Ito, Mitsuteru; Ferguson-Smith, Anne C; Kono, Tomohiro (2007). "High-frequency generation of viable mice from engineered bi-maternal embryos". Nature Biotechnology. 25 (nine): 1045–1050. doi:ten.1038/nbt1331. PMID 17704765. S2CID 7242745.
- ^ Kawahara, G.; Kono, T. (2009). "Longevity in mice without a father". Man Reproduction. 25 (2): 457–461. doi:ten.1093/humrep/dep400. PMID 19952375.
- ^ a b c d e Bischoff, S. R.; Tsai, S.; Hardison, N.; Motsinger-Reif, A. A.; Freking, B. A.; Nonneman, D.; Rohrer, G.; Piedrahita, J. A. (2009). "Characterization of Conserved and Nonconserved Imprinted Genes in Swine". Biology of Reproduction. 81 (5): 906–920. doi:10.1095/biolreprod.109.078139. PMC2770020. PMID 19571260.
- ^ Versieren, Thou; Heindryckx, B; Lierman, Due south; Gerris, J; De Sutter, P. (2010). "Developmental competence of parthenogenetic mouse and human being embryos after chemic or electrical activation". Reprod Biomed. 21 (6): 769–775. doi:10.1016/j.rbmo.2010.07.001. PMID 21051286.
- ^ a b Mori, Hironori; Mizobe, Yamato; Inoue, Shin; Uenohara, Akari; Takeda, Mitsuru; Yoshida, Mitsutoshi; Miyoshi, Kazuchika (2008). "Effects of Cycloheximide on Parthenogenetic Development of Pig Oocytes Activated by Ultrasound Treatment". Periodical of Reproduction and Development. 54 (5): 364–369. doi:10.1262/jrd.20064. PMID 18635923.
- ^ Time magazine, November 28, 1955; Editorial in The Lancet, 2: 967 (1955)
- ^ a b de Carli, Gabriel Jose, and Tiago Campos Pereira. "On human parthenogenesis." Medical Hypotheses 106 (2017): 57–lx.
- ^ Philip Cohen, "The boy whose blood has no father", New Scientist, vii.10.1995
- ^ Revazova, E.South.; Turovets, N.A.; Kochetkova, O.D.; Kindarova, Fifty.B.; Kuzmichev, L.North.; Janus, J.D.; Pryzhkova, G.Five. (2007). "Patient-Specific Stem Cell Lines Derived from Man Parthenogenetic Blastocysts". Cloning and Stem Cells. 9 (3): 432–449. doi:10.1089/clo.2007.0033. PMID 17594198.
- ^ Revazova, E.S.; Turovets, N.A.; Kochetkova, O.D.; Agapova, 50.Southward.; Sebastian, J.50.; Pryzhkova, M.V.; Smolnikova, V.Iu.; Kuzmichev, L.North.; Janus, J.D. (2008). "HLA Homozygous Stalk Prison cell Lines Derived from Human Parthenogenetic Blastocysts". Cloning and Stem Cells. 10 (1): xi–24. doi:10.1089/clo.2007.0063. PMID 18092905.
- ^ Williams, Chris. "Stem cell fraudster fabricated 'virgin birth' breakthrough: Silver lining for Korean science scandal", The Register, three August 2007.
- ^ Herring, Amy H.; Attard, Samantha K.; Gordon-Larsen, Penny; Joyner, William H.; Halpern, Carolyn T. (December 17, 2013). "Like a virgin (mother): analysis of data from a longitudinal, Usa population representative sample survey". BMJ. 347: f7102. doi:10.1136/bmj.f7102. ISSN 1756-1833.
- ^ "No sex for all-girl fish species". BBC.CO.UK. April 23, 2008. Retrieved 2017-06-11 .
- ^ a b c d eastward f g h Holsbeek, Chiliad.; Jooris, R. (2010). "Potential impact of genome exclusion by conflicting species in the hybridogenetic water frogs (Pelophylax esculentus circuitous)". Biological Invasions. 12: 1–xiii. doi:x.1007/s10530-009-9427-ii. ISSN 1387-3547. S2CID 23535815.
- ^ a b Schultz, R. Jack (November–Dec 1969). "Hybridization, unisexuality, and polyploidy in the teleost Poeciliopsis (Poeciliidae) and other vertebrates". The American Naturalist. 103 (934): 605–619. doi:10.1086/282629. JSTOR 2459036. S2CID 84812427.
- ^ Vrijenhoek, Robert C. (1998). "Parthenogenesis and Natural Clones" (PDF). In Knobil, Ernst; Neill, Jimmy D. (eds.). Encyclopedia of Reproduction. Vol. 3. Bookish Printing. pp. 695–702. ISBN978-0-12-227020-eight.
- ^ Simon, J.-C.; Delmotte, F.; Rispe, C.; Crease, T. (2003). "Phylogenetic relationships between parthenogens and their sexual relatives: the possible routes to parthenogenesis in animals" (PDF). Biological Journal of the Linnean Society. 79: 151–163. doi:10.1046/j.1095-8312.2003.00175.x . Retrieved 2015-06-21 .
- ^ Vrijenhoek, J Yard; J C Avise; R C Vrijenhoek (January 1, 1992). "An Aboriginal Clonal Lineage in the Fish Genus Poeciliopsis (Atheriniformes: Poeciliidae)". Proceedings of the National Academy of Sciences U.s.a.. 89 (1): 348–352. Bibcode:1992PNAS...89..348Q. doi:10.1073/pnas.89.ane.348. ISSN 0027-8424. PMC48234. PMID 11607248.
- ^ "Hybridogenesis in Water Frogs". tolweb.org.
- ^ Beukeboom, L. W; R. C Vrijenhoek (1998). "Evolutionary genetics and environmental of sperm‐dependent parthenogenesis". Journal of Evolutionary Biology. 11 (half-dozen): 755–782. doi:10.1046/j.1420-9101.1998.11060755.x. ISSN 1420-9101. S2CID 85833296.
- ^ Vorburger, Christoph; Reyer, Heinz-Ulrich (2003). "A genetic mechanism of species replacement in European waterfrogs?" (PDF). Conservation Genetics. 4 (2): 141–155. doi:ten.1023/A:1023346824722. ISSN 1566-0621. S2CID 20453910. Retrieved 2015-06-21 .
- ^ Inácio, A; Pinho, J; Pereira, PM; Comai, L; Coelho, MM (2012). "Global Analysis of the Small RNA Transcriptome in Unlike Ploidies and Genomic Combinations of a Vertebrate Complex – The Squalius alburnoides". PLOS One. 7 (seven: e41158): 359–368. Bibcode:2012PLoSO...741158I. doi:10.1371/journal.pone.0041158. PMC3399795. PMID 22815952.
- ^ Saitoh, K; Kim, I-S; Lee, E-H (2004). "Mitochondrial gene introgression betwixt spined loaches via hybridogenesis". Zoological Scientific discipline. 21 (7): 795–798. doi:10.2108/zsj.21.795. PMID 15277723. S2CID 40846660.
- ^ Mantovani, Barbara; Scali, Valerio (1992). "Hybridogenesis and androgenesis in the stick-insect Bacillus rossius-Grandii benazzii (Insecta, Phasmatodea)". Development. 46 (3): 783–796. doi:x.2307/2409646. JSTOR 2409646. PMID 28568678.
Further reading [edit]
- Dawley, Robert Chiliad. & Bogart, James P. (1989). Evolution and Ecology of Unisexual Vertebrates. Albany: New York Land Museum. ISBN 1-55557-179-4.
- Fangerau, H (2005). "Can bogus parthenogenesis sidestep ethical pitfalls in human therapeutic cloning? An historical perspective". Journal of Medical Ethics. 31 (12): 733–735. doi:10.1136/jme.2004.010199. PMC1734065. PMID 16319240.
- Futuyma, Douglas J. & Slatkin, Montgomery. (1983). Coevolution. Sunderland, Mass: Sinauer Associates. ISBN 0-87893-228-three.
- Hore, T; Rapkins, R; Graves, J (2007). "Construction and evolution of imprinted loci in mammals". Trends in Genetics. 23 (9): 440–448. doi:10.1016/j.tig.2007.07.003. PMID 17683825.
- Kono, T.; Obata, Y.; Wu, Q.; Niwa, K.; Ono, Y.; Yamamoto, Y.; Park, E.S.; Seo, J.-S.; Ogawa, H. (2004). "Nascence of parthenogenetic mice that can develop to adulthood". Nature. 428 (6985): 860–864. Bibcode:2004Natur.428..860K. doi:10.1038/nature02402. PMID 15103378. S2CID 4353479.
- Maynard Smith, John. (1978). The Evolution of Sex activity. Cambridge: Cambridge University Printing. ISBN 0-521-29302-2.
- Michod, Richard East. & Levin, Bruce R. (1988). The Evolution of Sex activity. Sunderland, Mass: Sinauer Assembly. ISBN 0-87893-459-vi.
- Schlupp, Ingo (2005). "The Evolutionary Ecology of Gynogenesis". Almanac Review of Environmental, Evolution, and Systematics. 36: 399–417. doi:10.1146/annurev.ecolsys.36.102003.152629.
- Simon, J; Rispe, Claude; Sunnucks, Paul (2002). "Ecology and evolution of sex in aphids". Trends in Ecology & Evolution. 17: 34–39. doi:10.1016/S0169-5347(01)02331-X.
- Stearns, Stephan C. (1988). The Evolution of Sex and Its Consequences (Experientia Supplementum, Vol. 55). Boston: Birkhauser. ISBN 0-8176-1807-4.
External links [edit]
- Reproductive beliefs in whiptails at Crews Laboratory
- Types of asexual reproduction
- Parthenogenesis in Incubated Turkey Eggs from Oregon State Academy
- National Geographic NEWS: Virgin Nascence Expected at Christmas – By Komodo Dragon
- BBC NEWS: 'Virgin births' for behemothic lizards (Komodo dragon)
- REUTERS: Komodo dragon proud mum (and dad) of five
- Female sharks capable of virgin birth
- Scientists confirm shark'due south 'virgin birth' Article by Steve Szkotak AP updated ane:49 a.m. ET, Friday., Oct. 10, 2008
Source: https://en.wikipedia.org/wiki/Parthenogenesis
Posted by: craftratepand.blogspot.com

0 Response to "What Animals Reproduce By Having Their Babies Grow Out Of Their Sides"
Post a Comment